1) Legal Basis of new food raw material
Companies who plan to use new food raw materials to manufacture food products shall submit the related safety assessment material of the new food raw material to National Health Commission of the People’s Republic of China (former NHFPC).
Food Safety Law of the People‘s Republic of China (2015 version)
2) Applicant
Any entity or individual that intends to engage in production, use or import of a new food raw material.
3) Registration procedure of new food raw material
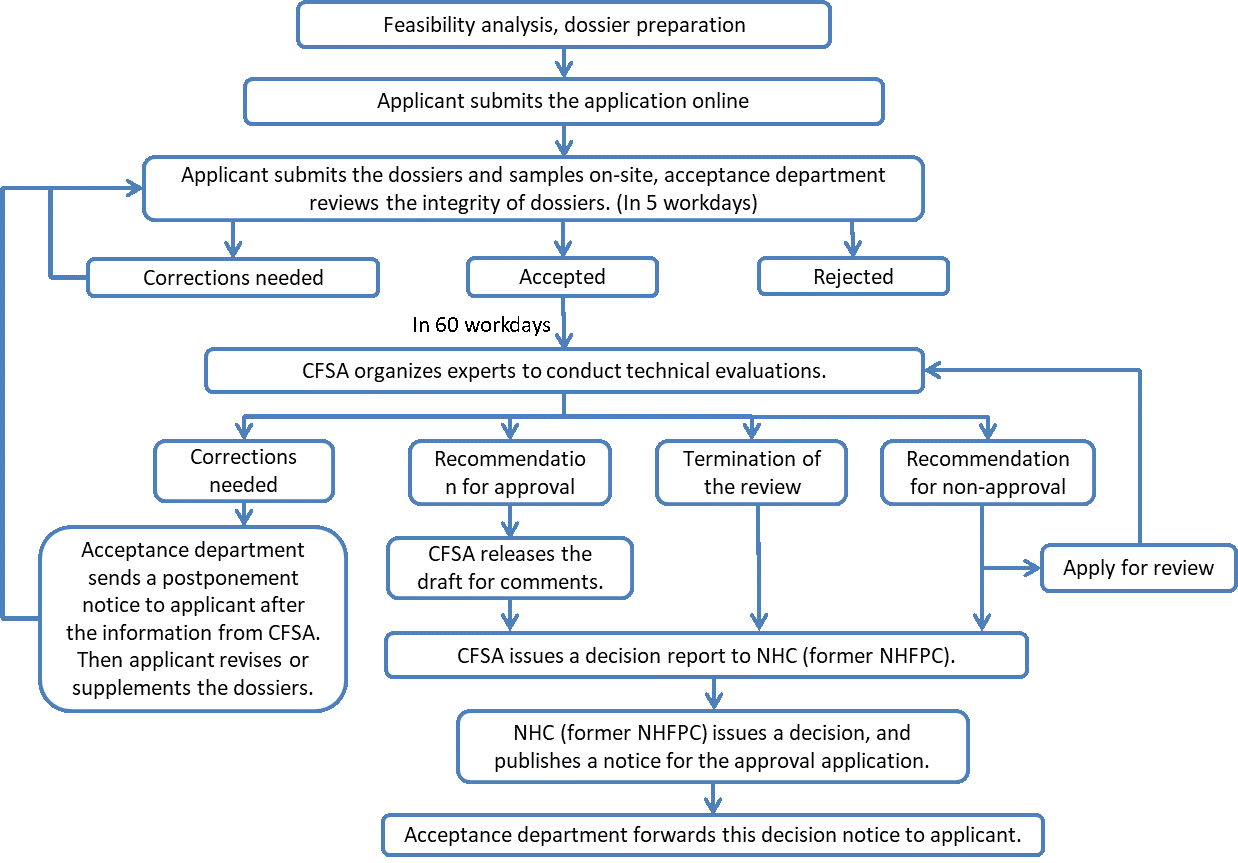
4) Dossier Requirements of new food raw material registration
• Application form
• Research and development report on the new food raw material.
• Safety evaluation report.
• Production techniques
• Relevant implemented standards applied to the material (including safety requirements, quality specifications and inspection measures).
• Information on utilization thereof and domestic and international research thereon as well as relevant safety evaluation materials.
• Other materials conducive to evaluation and review.
• In addition, the applicant shall attach one sealed product sample or 30 grams of the raw material.
5) Additional required documents for export new food raw materials
• Supporting documents issued by relevant authority or institution in the exporting country (region) allowing such product to be produced or sold in the exporting country (region).
• Supporting documents for review or certification of the manufacturer issued by relevant institution or organization in the country (region) where the manufacturer is located.
6) Product test scope
• Components analysis report
• Hygiene test report
• Toxicology safety assessment documents
• Microbial resistance test report and toxigenicity test report
• Safety assessment
• Other test (if necessary) (e.g. method verification)
1. Duration of new food raw material registration
|
Main procedures |
Estimated Time |
|
Composition analysis tests and Hygiene inspection tests |
14 workdays |
|
Toxicity tests |
5-10 months |
|
Safety assessment report |
Usually within 3 months |
|
Application acceptance |
5 workdays |
|
Technical review |
60 workdays |
|
Re-evaluation if necessary (supplement dossiers) |
1 year |
|
CFSA issues the draft approval for comments |
1 month |
|
NHC makes the final approval decision |
Within 20 workdays |
|
Total |
15-25 months |