Export procedures
Non-special-purpose Cosmetics Filing Procedure
1. Legal basis of Non-special-purpose Cosmetics Filing
For finished cosmetics, companies who plan to place cosmetics on Chinese market must apply for and obtain hygiene license or record-keeping certificate from the China Food & Drug Administration(CFDA). Foreign companies shall appoint a Chinese responsible agent to deal with registration and obtain such certificate. Manufacturers shall also register a new cosmetic ingredient prior to using it for cosmetics production.
2. Application qualification
Generally qualified as a foreign cosmetics brand owner.
Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been put on the market.
If the products produced exclusively for the Chinese market and cannot provide documents for sale in the producing country (region) or country (region) of origin, relevant research and experimental data for Chinese consumers shall be submitted alternatively.
3. Application procedure
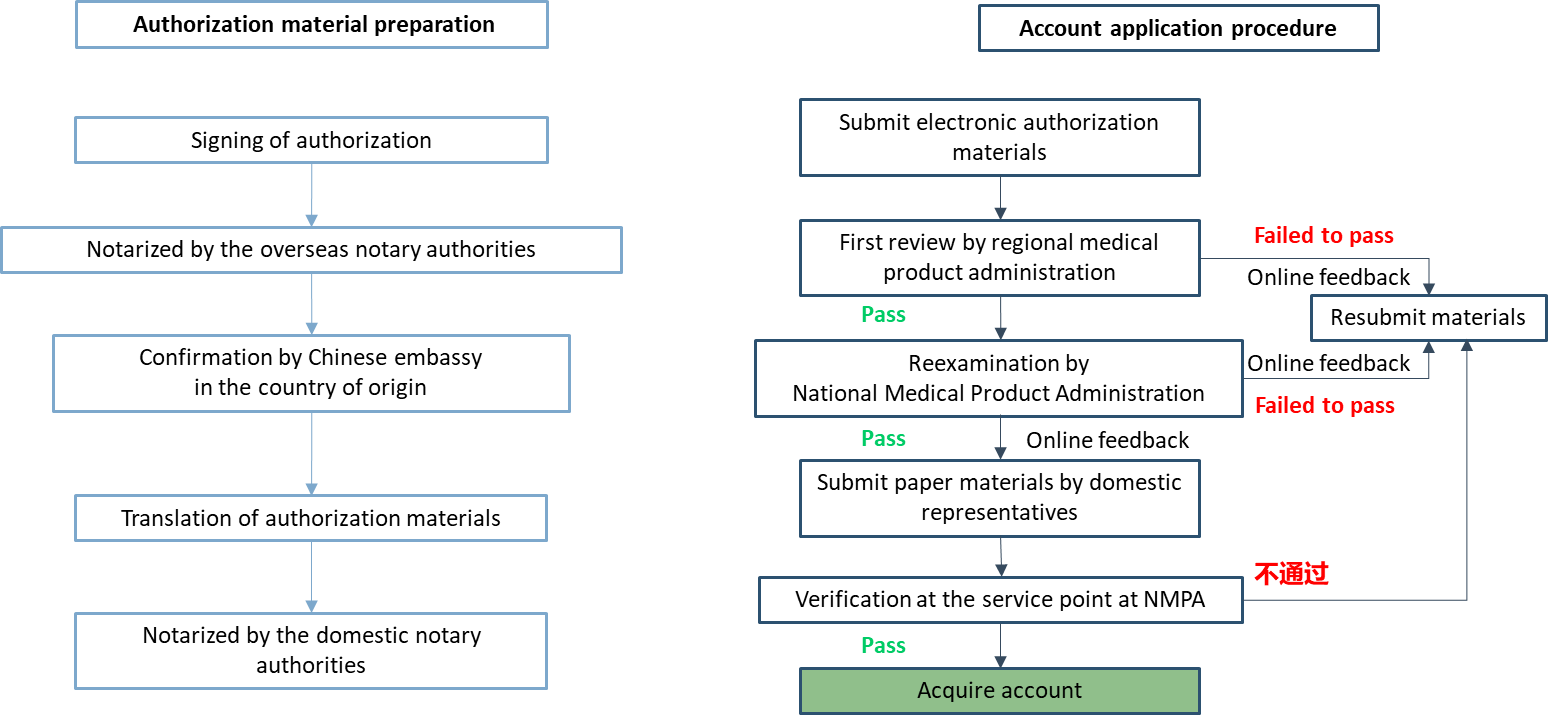
Application for account
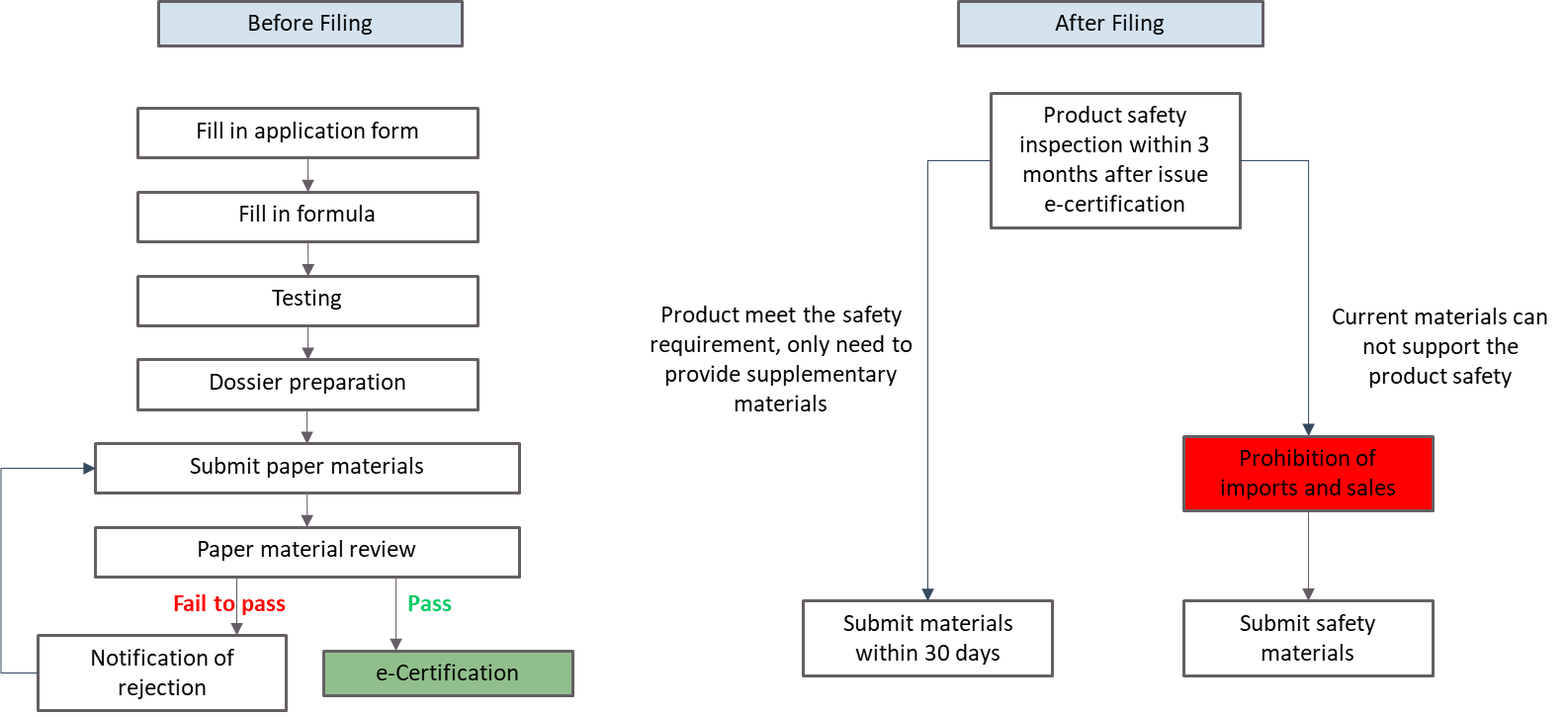
Filing prodecure
4. Dossier requirements of non-special-purpose cosmetics registration
- Application form for license (online filing)
- Naming basis of product Chinese name
- Product formula (online filing)
- Manufacturing technique and diagram
- Product quality control requirement
- Product package (sales package & product label);
- Testing report from a cosmetics testing institution approved by the SFDA and related materials
- Relevant safety assessment data on risk substances in products
- Products for hair growth, breast shaping and fitness, scientific documents on functional ingredients and relative usage references need to be submitted.
- For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided
- The statement on related problems of ‘Mad Cow Disease’
- Certified document for production and sales in the manufacturing country (region)
- Product technique requirement (online)
- Relevant proving materials for overseas enterprises on production quality management
- Other documents that may be helpful for inspection
5. Testing Requirement
- Physiochemical testing
- Microbiological testing
- Toxicity testing
6. Filing Duriation
| Main Procedure |
Estimated duration |
| Testing duration |
40-45 working days |
| Filing duration |
4-5 months |
| Total |
6-7 months |
Special-purpose cosmetics registration
1. Legal basis of health food registration
For finished cosmetics, companies who plan to place cosmetics on Chinese market must apply for and obtain hygiene license or record-keeping certificate from the China Food & Drug Administration(CFDA). Foreign companies shall appoint a Chinese responsible agent to deal with registration and obtain such certificate. Manufacturers shall also register a new cosmetic ingredient prior to using it for cosmetics production.
2. Applicant qualification for registration
- Generally qualified as a foreign cosmetics brand owner.
- Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been put on the market.
- If the products produced exclusively for the Chinese market and cannot provide documents for sale in the producing country (region) or country (region) of origin, relevant research and experimental data for Chinese consumers shall be submitted alternatively.
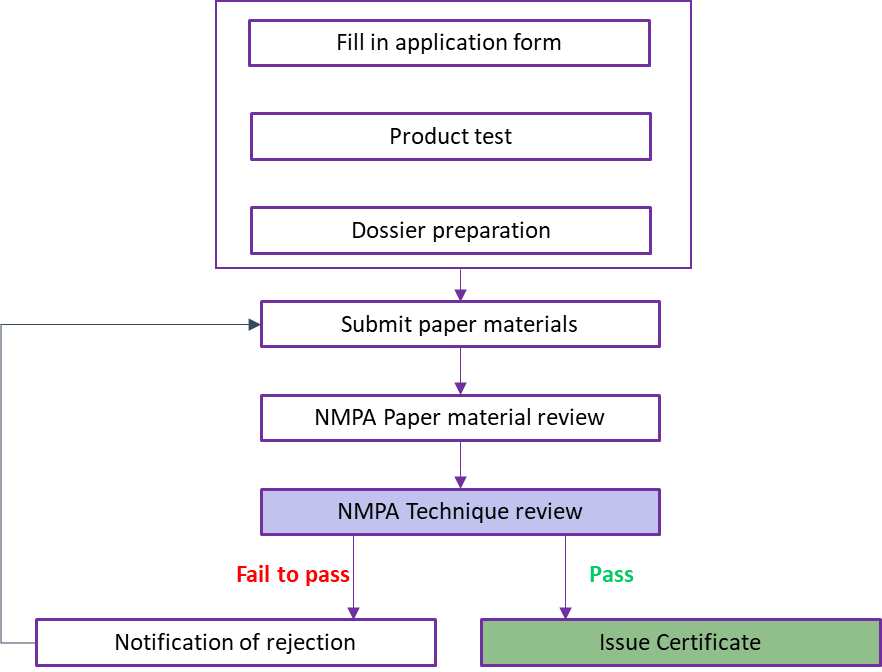
3. Registration Procedure
4. Dossier requirements of Special-purpose Cosmetics Registration
- Application form for license (online filing)
- Naming basis of product Chinese name
- Product formula (online filing)
- Manufacturing technique and diagram
- Product quality control requirement
- Product package (sales package & product label)
- Testing report from a cosmetics testing institution approved by the SFDA and related materials
- Relevant safety assessment data on risk substances in products
- Products for hair growth, breast shaping and fitness, scientific documents on functional ingredients and relative usage references need to be submitted.
- For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided
- The statement on related problems of ‘Mad Cow Disease’
- Certified document for production and sales in the manufacturing country (region)
- Product technique requirement (online)
- Relevant proving materials for overseas enterprises on production quality management
- Other documents that may be helpful for inspection
5. Testing Requirement
- Physiochemical testing
- Microbiological testing
- Toxicity testing
- Safety evaluation of using tests of cosmetics on human body
6. Registration Duration
Baced on each product, the registration duration will vary


