1) Legal Basis of new food additive registration
Companies who plan to use new food additives (including enlarge the usage scope of existing food additives) or import new food additives to Chinese market shall submit the related safety assessment material to National Health Commission of the People's Republic of China (former NHFPC).
Food Safety law of the people’s republic of China (2015 version)
2) Applicant
Any entity or individual that intends to engage in production, use or import of a new food raw material.
3) Registration procedure of new food additive
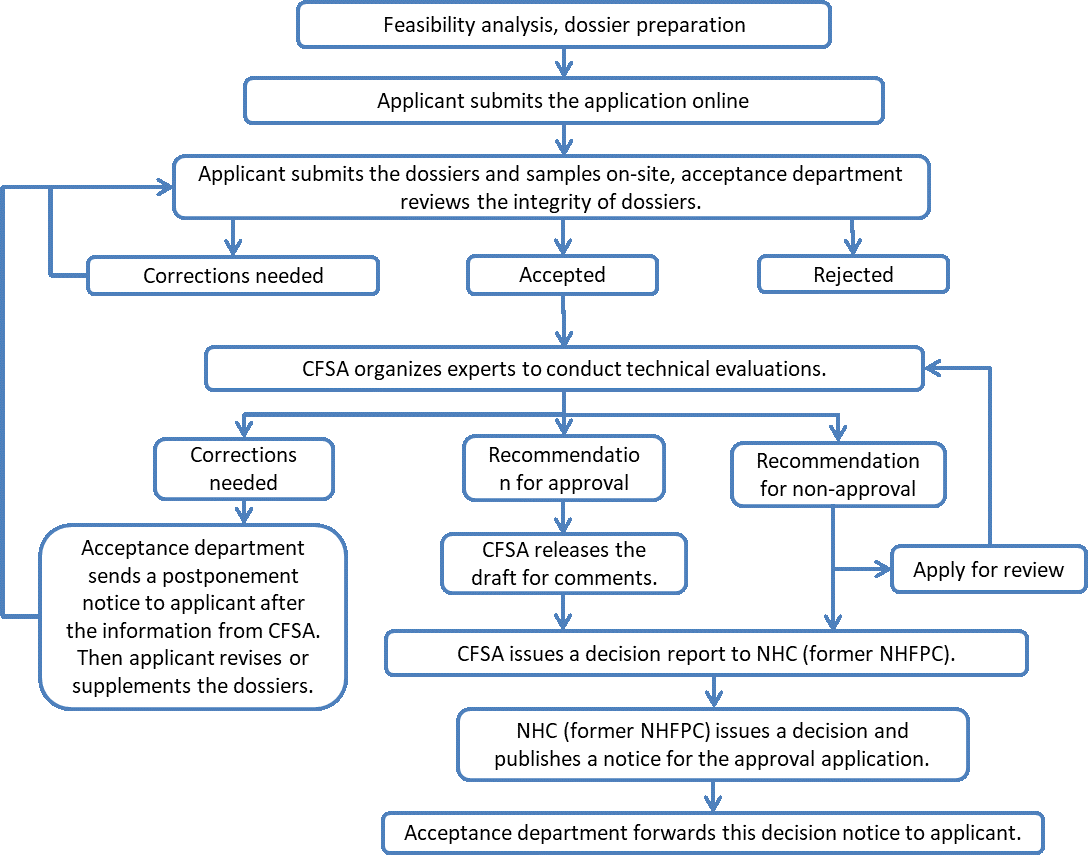
4) Dossier requirements of new food additive registration
• Application form
• Generic name, functional classification, dosage and range of use of the additive at issue;
• Materials or documents proving its technical necessity and effects
• Quality and specifications requirements, manufacturing techniques and inspection methods of food additives, the inspection methods for the additive in question, and other relevant information;
• Safety assessment materials(when apply for expanding the usage scope or dosage, this material can be exempted)
• Label and manual.
• Other materials that contribute to the safety review, such as legal permission of producing and using from other countries (regions) or international organizations
• Declaration of power of attorney (when the registration application run by entrusted agencies)
5) Additional required documents for export new food additive
• Supporting documents issued by relevant authority or institution in the exporting country (region) allowing such product to be produced or sold in the exporting country (region).
• Supporting documents for review or certification of the manufacturer issued by relevant institution or organization in the country (region) where the manufacturer is located.
6) Product test scope
• Quality specification test report
Three batches of new food additive shall be tested according to the quality specifications and inspection methods.
• Toxicity safety assessment
i. Toxicity tests shall be proceeded according to GB 15193.1-2014.
ii. Replace option: Toxicological evaluation materials of this food additive from oversea GLP laboratory can replace the tests.
Duration of new food additive registration
| Main Procedure | Estimated duration | |
| Qualification specification tests | 14 workdays | |
| Toxicity tests (if necessary) | 5-10 months | |
| Application acceptance | 5 workdays | |
| Technical review | 60 workdays | |
| Re-evaluation if necessary (supplement dossiers) | 1 year | |
| CFSA issues the draft approval for comments | 1 month | |
| NHC makes the final approval decision | Within 20 workdays | |
| Total | For expanding the usage scope or dosage | 8-10 months |
| For new food additive registration | 12-22 months | |