(一) Factory registration
1. Reference of registration
According to Food safety law of the People’s republic of China, Measures for the Supervision and Administration of Inspection and Quarantine of Imported and Exported Dairy Products (Order of the General Administration of Quality Supervision, Inspection and Quarantine No. 152), Provisions on the Administration of Registration of Foreign Enterprises Producing Imported Food (Order of the General Administration of Quality Supervision, Inspection and Quarantine No. 145) and Announcement on Publishing the Catalogue for the Implementation of Registration of Overseas Enterprises Producing Imported Food (Announcement No. 62 [2013] of the General Administration of Quality Supervision, Inspection and Quarantine), the foreign food production enterprises in The Catalogue will not able to import their products to China until they finish their registration. The Catalogue is at follows:
2. 注册条件Conditions of registration
1) Relevant veterinary service system, plant protection system and public health management system of the country (region) in which the enterprise is located should be qualified after evaluation.
2) The animal and plant raw materials used in food exported to China should come from non-infected area. If the products are suspected to have risks of infecting animal and plant diseases, the local administrative authority should provide with relevant document to demonstrate the elimination or controllability of the risks.
3) With the approval and inspection of local administrative authorities, the sanitary condition should meet the requirements of Chinese laws and regulations.
3. Application procedures
If the food enterprises intend to apply for the registration of import, they should be recommended to the General Administration of Customs of the People's Republic of China (GACC )by the competent authority of the country (region) in which enterprises are located, as well as submitting proving documents and materials.
4. Requirements of application documents
1) Written document of relevant laws and regulations from local countries (regions) in which the enterprise is located, including epidemic situation, veterinary sanitary situation, public sanitary situation, plant protection, pesticide and drug residues, registration administration of the food producing enterprises, and sanitary requirements.
Written document of the institutional and personnel setup of competent authority from local countries (region) in which the enterprise is located, and its execution of laws and regulations.
2) List of overseas food production enterprises applying for registration.
3) Evaluation to the quarantine and sanitary controlling situation of recommended enterprise by the competent authority from local countries (region) in which the enterprise is located.
4) Announcement of requiring the recommended enterprise to observe Chinese laws and regulations by competent authority from local country (region) in which the enterprise is located.
5) Application form of the enterprise, and also the floor map of factories, workshops and freezers, flow diagrams, if necessary.
(二) Formula registration
1. Reference of registration
According to Food Safety Law of the People’s Republic of China, the food and drug administration has published Administrative Measures for the Registration of Product Formulas of Infant Formula Milk Powder, which has been implemented since 01.10.2016. The aim of the Measures is to strengthen the administration of infant milk powder product registration and guarantee the quality and safety of products.
2. Conditions of registration
1) The applying enterprises, including enterprises which intend to produce and sell the infant formula milk power products in China and also the foreign enterprises which intend to export the infant formula milk powder products to China, should equip with relevant R&D, production and inspection capabilities.
2) According to the national food safety standard and relevant laws and regulations, the applying enterprises should observe the requirements of manufacturing practice for powdered formulas for infants and young children, implement HACCP system and batch-by batch inspection.
3. Division of Responsibility
ü State Administration of Market Regulation is responsible for the administration of infant milk powder product registration.
ü General Administrative Agency (General administrative business acceptance and complaint reporting center) is responsible for the acceptance of registration application.
ü General Food Reviewing Agency (General Health Food Reviewing Agency) is responsible for the evaluation of registration application.
ü General inspection agency (General food and drug inspection center) is responsible for the on-site verification of the registration application.
4. Application procedure
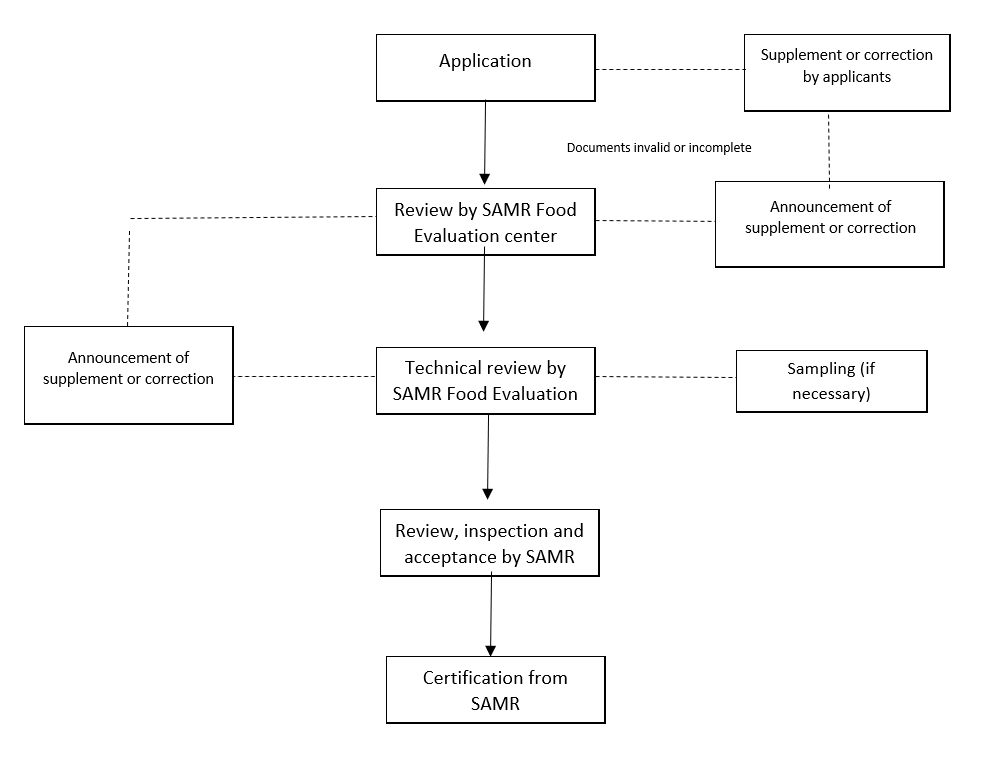
5. Requirements of application documents
1) List of application documents:
A. Application form for product formulas of infant formula milk powder registration
B. Qualification documents of applicants
C. Quality and safety standard of raw materials
D. Product formula
E. Product formula R&D demonstration report
F. Process flow description
G. Product examination report
H. Proving document related to R&D, product and inspecting capabilities
I. Label, specification and relevant proving documents
2) General requirements of application documents
A. Document should be printed in A4 size paper (Chinese characters should not be smaller than 12pt in Song typeface, English characters should not be smaller than 12pt.)
B. Applicants should submit the original document with five copies and digital one at the same time. If the applicants are required to supplement the documents during the evaluation, applicants should provide the original document with four copies and also the digital document.
3) Specific requirements of the registration application
A. The application document should be stamped with the official seal of applicants at every page or the edge of two sheets of paper (except the application form and inspecting report drawn by inspecting institutions). If the applicants are staying overseas without the stamp, the document should be stamped with the official seal of the representative office or domestic agency in China. The seal should be stamped on the text.
B. The information on the application document, such as the name, address, legal representative, should be correspond to the qualification document of the applicants. The information on the application document (such as name, address, product name, etc.) should be consistent. The stamp on the document should be correspond to the name of applicants (except for the seal of representative office or domestic agency in China).
C. The foreign language in the application document (including qualification document, quality and safety standard of raw materials, product formula, process flow description, inspection report, label and specification, etc.) should be all translated into standard Chinese. The abstract from foreign language references and keywords and other contents which are relevant to the scientificity and safety of formula should be translated into standard Chinese (except for names and addresses). Applicants should guarantee the authenticity, accuracy and consistency of the translated document.
6. Formula modification application document & general requirements
A. Application form for modification of product formulas of infant formula milk powder
B. Registration certificate of product formulas of infant formula milk powder, as well as the copied document.
C. Proving document relevant to formula modification.
(1)Overseas applicants which intend to entrust for modification should submit the relevant proving documents of entrustment based on the requirements of registration.(2)Applicants should provide the relevant copies of qualification documents, such as business license, organization code and overseas applicant registration qualification, etc.(3)Applicants should list the modified items, reasons for modification and relevant references. ① While applying for modification of product name, the modified name should comply with the relevant naming regulations. ② While applying for modification of enterprise, address and legal representatives, applicants should submit the relevant proving document from the government in which the enterprise is located. ③ While applying for modification of formula, applicants should list the modified part and the formula after modification. Applicants should come up with the verification report related to the necessity, safety and scientificity of modification. If the modification will influence the scientificity or safety of the product, applicants should submit the application document based on the initial application requirements.
7. Formula renewal registration documents & project general requirements
A. Application form of formula renewal for infant formula milk powder products
B. Copies of qualification document of applicants
C. Relevant document related to R&D, production and inspection capabilities of the enterprise.
D. Self-inspection report related to quality management of enterprises.
E. Tracing evaluation of nutrition and safety management in the recent five years, including production, sales, sampling (by inspection institution) and self-inspection situation, as well as the analysis of defected products. Applicants should also provide the analysis report related to the product consumption and follow-up evaluation from the market, as well as the research and control instructions of hazardous components from the raw materials and supplements.
F. Opinion letter from the market supervision and administration agencies of the provinces, municipalities and autonomous regions in which the enterprise is located.
G. Registration certificate of product formulas of infant formula milk powder, in addition to the copied document.
(三) Filings of imported foods
1. Reference
1) “Measures for the Safety Administration of Imported and Exported Food”, (Order by General Administration of Customs No.243), Article 19, “ The inspection and quarantine institution shall apply a filing management system to food importers.”
2) “Measures for the Inspection and Quarantine Administration of Imported and Exported Food”(Former Order of General Administration of Quality Supervision, Inspection and Quarantine No. 144, 2012)
3) Announcement on publishing ‘Regulations for Exported Foods Filing Administration’ and ‘Regulations for Exported Filings and Sales Records Administration of Food products’” (Former Order of General Administration of Quality Supervision, Inspection and Quarantine No. 55, 2012)
4) “Announcement of updating the export and import filing system of imported foods” (Former Announcement of General Administration of Quality Supervision, Inspection and Quarantine No. 98, 2015)
2. Filing conditions
1) Submitted documents should be true, complete and effective.
2) The qualified business scope of the enterprise should include product manufacturing, distributing and agent of foods or cosmetics products (goods). Enterprises should also be qualified by the recent annual inspection of the market supervision and administration agencies.
3) For the foreign-funded enterprises, they should be qualified by the recent joint annual inspection.
4) Enterprises should have fixed officed space in the regions which has registered in market supervision and administration agencies.
5) Enterprises should establish and implement effective foods quality and safety management regulations
6) Enterprises should cooperate with customs in performing official duties, avoid shrinking the relevant responsibilities.
7) Enterprises should have no violations to laws or regulations.
3. Responsible department
Custom in the place of registration
4. Filing documents
1) Application form of filing (see attachment).
2) Information of institutional setup, departmental responsibilities and occupational responsibilities of agencies which take charge of food safety management.
3) Varieties of food products which intend to sell in the market, and locations of storage.
4) Relevant statement of importing, manufacturing and selling situation of food and cosmetic products of the enterprise in recent two years.
5. filing procedures
1) Login to “Registration Systems of Imported Food and Cosmetic Importers and Exporters”(the system has been integrated with “Internet+ Custom” platform) and submit the relevant information.
2) Enterprises should submit the prepared documents to local custom offices. Enterprises should be responsible to the integrity, effectiveness and accuracy to the submitted documents, and ensure that relevant personnel on the filled information can be contacted while meeting the case of emergency.