1) Legal basis of health food filing
Companies who plan to place health food in Chinese market shall apply and obtain the health food registration certificate or filing certificate. —— Food Safety law of the people’s republic of China (2015 version)
2) Applicant qualification for filing
• For imported health food, the filing applicant could be the oversea manufacturer of the marketed health food (oversea manufacturer refers to the legal person or other organization).
• Filing applicant of imported health food shall be the owner of the product, while manufacturer of the product is the actual producer (if the applied product is produced by the applicant, the manufacturer filled in the application form is the applicant; if the applied product is produced by overseas enterprises upon entrustment, the manufacturers shall be entrusted enterprises).
3) Procedure of health food export
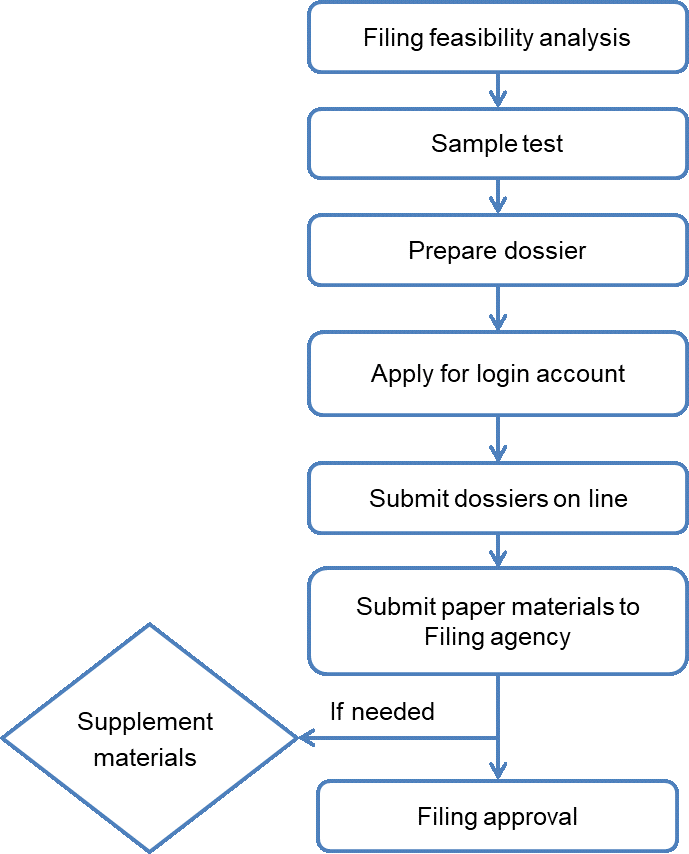
4) Dossier requirements of health food filing
• Health food filing application form; Letter of commitment for authenticity of the materials;
• Copies of legally registered certificates of the applicant;
• Product formulation materials;
• Product production process materials;
• Safety and function assessment material;
• Information of packaging materials in direct contact with the product;
• Samples of product label and package insert;
• Product technical requirements;
• Test according to product technical requirements;
• Product name materials
• Other materials proving product safety and health function.
5) Additional required documents for export health food
• Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the registration applicant is the oversea manufacturer of the health food marketed;
• Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year similar to health food ;
• Health food-associated standards issued by the product producing country (region) of origin or international organizations;
• Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
• For filing affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for filing affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
6) General requirements for imported product dossier
• The Chinese translation of foreign proof documents and foreign label and package insert shall be notarized by Chinese notary authorities and in accordance with the original content;
• Any proof documents, authorization letter (agreement) etc. issued by oversea institute shall be the original copy with the official language of the country of origin and company′s seal or legal representatives (or authorized person) signature. The original copy shall be notarized by notary authorities and confirmed by the Chinese embassy in the country of origin.
• The authorization letter shall specify filing applicant, the name of the company who was authorized, product name, commitment and issue date.
7) Product test scope
A. Functional components
B. Hygiene test
C. Stability test
D. All items listed in product technical requirements
E. Other tests if necessary
Health Food Registration
1) Legal basis of health food registration
All imported health food (excluded nutrition supplement of which the vitamins and (or) minerals meet the requirements in the Health Food Raw Materials Directory) — Food Safety law of the people’s republic of China (2015 version)
2) Applicant qualification for registration
• For imported health food, the filing applicant could be the oversea manufacturer of the marketed health food (oversea manufacturer refers to the legal person or other organization).
• Filing applicant of imported health food shall be the owner of the product, while manufacturer of the product is the actual producer (if the applied product is produced by the applicant, the manufacturer filled in the application form is the applicant; if the applied product is produced by overseas enterprises upon entrustment, the manufacturers shall be entrusted enterprises).
3) Registration procedure
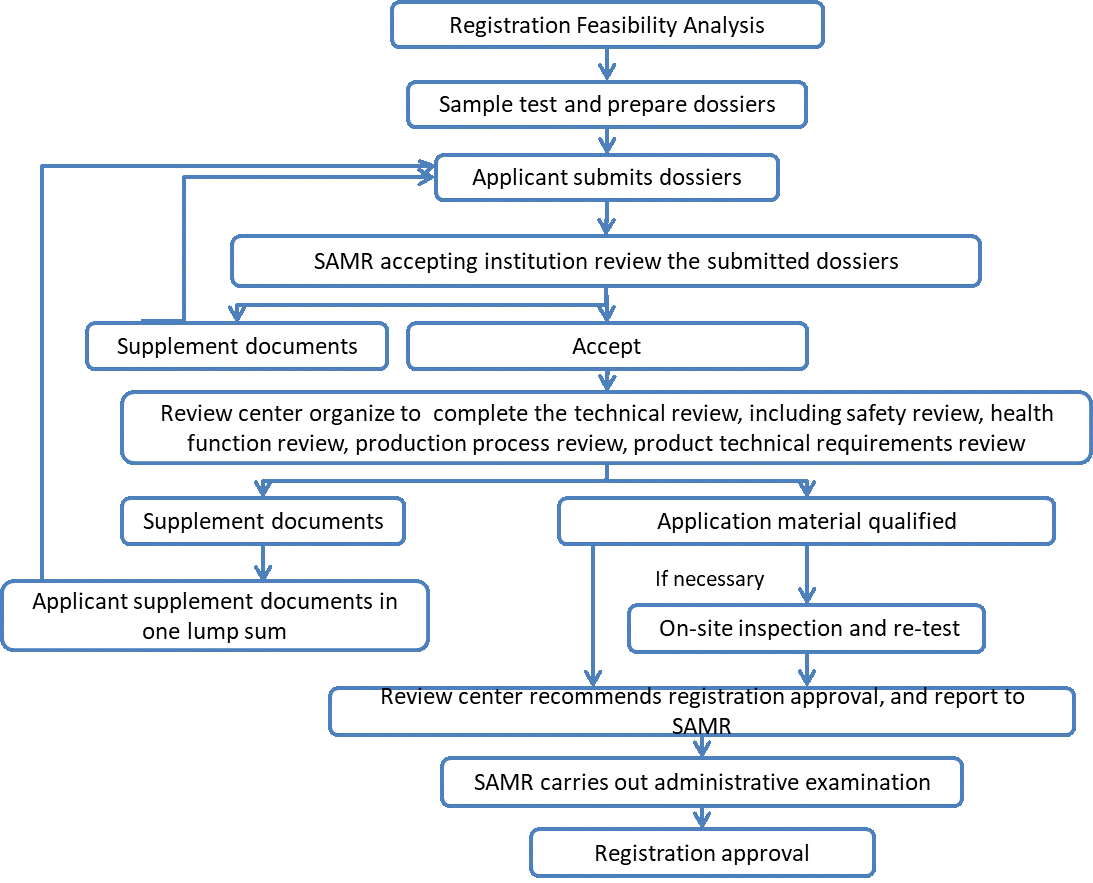
4) Dossier requirements of health food registration
• Health food registration application form; Letter of commitment for authenticity of the materials;
• Copies of legally registered certificates of the applicant;
• Product R&D report, including pilot scale validation data, argument report and relevant scientific evidences about the safety, health function, and quality controllability of the product and raw materials out the scope of raw material directory, product technical requirements, etc.;
• Product formulation materials (APIs and excipients);
• Product production process materials, including process flow diagram, CCP, etc. ;
• Safety and function assessment material;
• Information of packaging materials in direct contact with the product;
• Samples of product label and package insert;
• Retrieval materials show that the general name of the product is not in duplicate of registered drugs and the product name is not in duplicate of registered health foods;
• 3 samples with the minimum sales packaging;
• Other materials pertaining to the product registration technical evaluation.
5) Additional required documents for import health food
• Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the registration applicant is the oversea manufacturer of the health food marketed;
• Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year;
• Health food-associated standards issued by the product producing country (region) of origin or international organizations;
• Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
• For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for registration affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
6) General requirements for the submitted dossiers of imported product
• The Chinese translation of foreign proof documents and foreign label and package insert shall be notarized by Chinese notary authorities and in accordance with the original content;
• Any proof documents, authorization letter (agreement) etc. issued by oversea institute shall be the original copy with the official language of the country of origin and company′s seal or legal representatives (or authorized person) signature. The original copy shall be notarized by the notary authorities and confirmed by the Chinese embassy in the country of origin.
• The authorization letter shall specify registration applicant, the name of the company who was authorized, product name, commitment and issue date.
7) Product test scope
A. Functional components
B. Hygiene test
C. Stability test
D. All items listed in product technical requirements
E. Other test if necessary (such as safety assessments of health food new raw materials, strain virulence testing reports, etc.).
F. Self-test report
G. Methodological verification on Functional components test
H. Toxicology test
I. Animals function and (or) human function test
Duration of export health food to China
| Main procedures | Estimated duration |
| Feasibility analysis | 5 workdays |
| Test sample preparation | Case by case |
| Tests | 4 months |
| Dossier preparation and submission | 1 month |
| Accept the application by the accepting institution | 1 workday |
| Submit supplement dossiers | Case by case(normally 1 month) |
| Make approval decision, and issue the certificate | Case by case(1-4 weeks) |
| Total | 6-8 months |
Registration duration estimation
| Main procedures | Estimated duration |
| Feasibility analysis | 7 workdays |
| Test sample preparation | Case by case |
| Tests | 8-16 months |
| Dossier preparation and submission | 2-3 months |
| Accept the application by the accepting institution | 5 workdays |
| Transfer materials to review center | 3 workdays |
| SAMR technical review | 60 workdays |
| On-site inspection and re-test | Case by case for imported products |
| Submit supplement dossiers | Within 3 months |
| Report to SAMR | 5 workdays |
| Make approval decision, and issue the certificate | 33 workdays |
| Total | 2-3 years |