Formula registration
1. Reference
Article No.80 of Food Safety Law of the People's Republic of China,” Food for special medical purposes should be registered to the Food and Drug Administration Agency under the State Council”.
2. Conditions
1) Applicants should be manufacturing enterprises which intend to produce and sell the formula food for special medical purposes, or the foreign manufacturing enterprises which intend to export the food for special medical purposes to China.
2) Applicants should equip with relevant R&D capabilities and establish product R&D department with relative food-related senior professional specialists (or staffs with relevant professional capabilities).
3) Applicants should equip with relevant manufacturing capabilities, with food safety management and food-related professional specialists to implement the food safety management system and Good manufacturing practice for Food for Special Medical Purposes.
4) Applicants should equip with the relevant capabilities to take batch-by-batch inspection for products based on Good manufacturing practice for Food for Special Medical Purposes.
3. Responsible departments
ü State Administration of Market Regulation is responsible for the administration of infant milk powder product registration.
ü General Food Reviewing Agency (General Health Food Reviewing Agency) is responsible for the evaluation to the application.
ü General inspection agency (General food and drug inspection center) is responsible for the on-site verification of the registration.
4. Application procedures
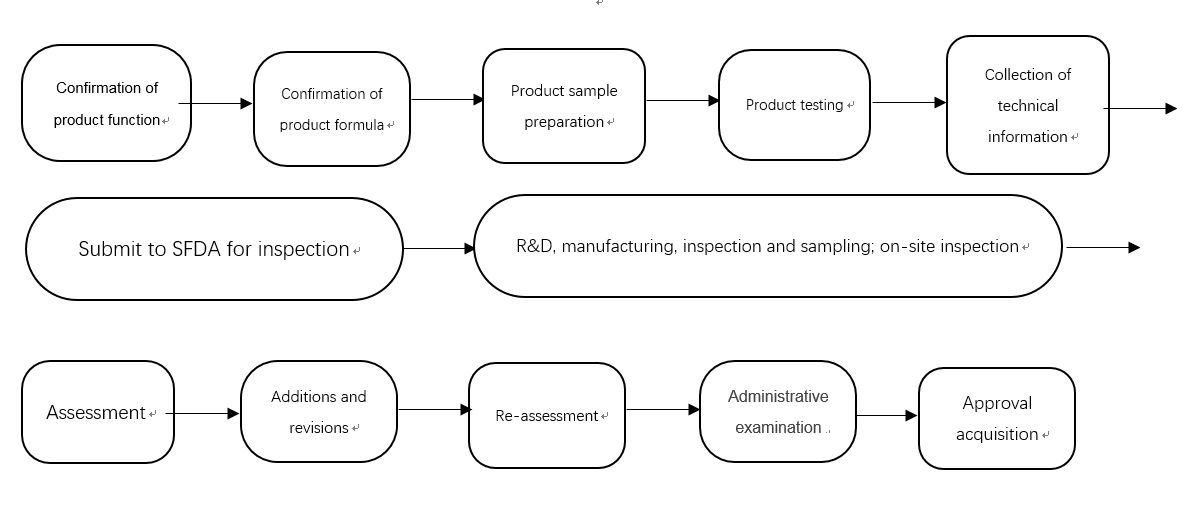
5. Required application documents for new product registration
1) Application form for new product registration
2) Product formula R&D and designing report, as well as the related references
3) Description of process flow and materials
4) Regulations of product standards
5) Label, specification and relevant proving documents
6) Test sample inspection report
7) Proving document related to R&D, product and inspecting capabilities
8) Documents related to product safety, nutritional adequacy and clinical effectiveness of special medical use.
9) Clinical trailing report (If applicants intend to apply for the product registration for full nutritional formula for specific needs)
10) Relevant approval documents related to application for registration
6. Formula renewal registration requirements
1) Application form of formula renewal for formula food for special medical purposes.
2) Registration certificate of the product, in addition to the copied document.
3) Copies of qualification document of applicants
4) Safety and quality management report of formula food for special medical purposes.
5) Self-inspection report of product quality management system.
6) Tracing evaluation to the product of formula food for special medical purposes.
7) If there are any modifications on the renewal registration compared to the previous one, applicants should give a clear identification of the changes, as well as submitting the relevant documents.
8) Research reports of the complete and long-term stability test research, which has been promised to hand over during application procedure
7. Change registration material requirements
1) Application form for modification of formula food for special medical purposes.
2) Registration certificate of product and relevant documents, as well as the copied documents.
3) Copies of qualification document of applicants
4) Proving documents that the product has been manufactured and sold, or explanations for the reason of not manufacturing or selling the product.
5) Introductions to the modified part, as well as the reasons and references for the modification.
For points 6, 7, and 8, the following information is also required for imported products:
a) Copies of qualification documents/certificates of foreign applicants from competent authorities or legal service agencies of countries (regions) in which the products are manufactured, as well as the Chinese translation.
b) Qualification documents of product permission of launching from competent authorities or legal service agencies of countries (regions) in which the products are manufactured, as well as the Chinese translation. The documents are not necessary if the products have not yet launched into the market.
c) Qualification documents to demonstrate that the enterprises have comply with the relevant quality management regulations.
d) If the registrations are handled by the resident agencies of foreign applicants in China, the agencies should submit the Certificate of Resident Agencies of Foreign Countries in China.
e) Foreign applicants which entrust the domestic agencies to handle the registration application should submit the notarized Power of Attorney document with Chinese translation, as well as the copy of business license of the entrusted agency.
8. Lead time of new product registration
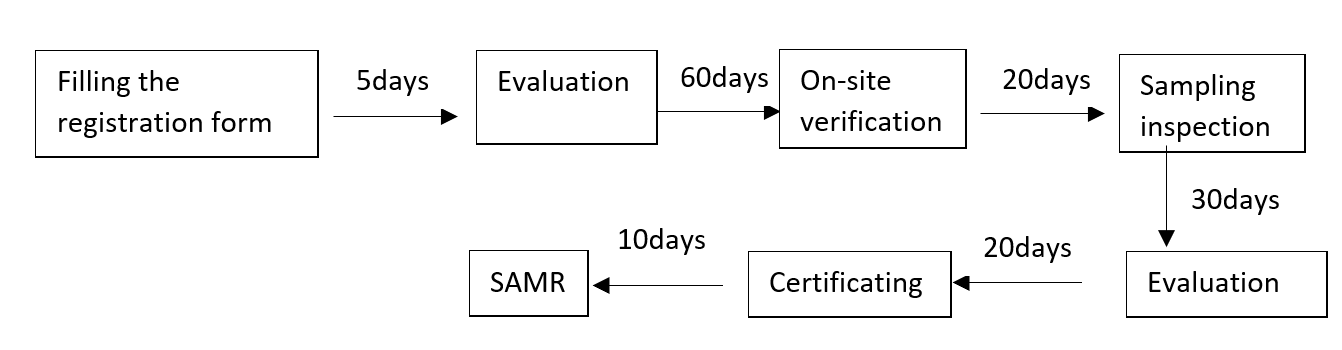
(二) Filing of the importer
1. References
1) Article No. 19 of Measures for the Safety Administration of Imported and Exported Food (Order of the State Administration of Quality Supervision, Inspection and Quarantine No. 144), ” The inspection and quarantine institution shall apply a filing management system to food importers.”
2)“Measures for the Inspection and Quarantine Administration of Imported and Exported Food”(Former Order of General Administration of Quality Supervision, Inspection and Quarantine No. 144, 2012)
3)“Announcement on publishing ‘Regulations for Export Foods Filing Administration’ and ‘Export Foods Filing and Regulations for Sales Filing Administration’” (Former Order of General Administration of Quality Supervision, Inspection and Quarantine No. 55, 2012)
4) “Announcement of updating the export and import filing system of imported foods” (Former Announcement of General Administration of Quality Supervision, Inspection and Quarantine No. 98, 2015)
2. Filing conditions
1) Submitted documents should be true, complete and effective.
2) The business scope of the enterprises’ qualifications should include product manufacturing, distributing and agent of foods or cosmetics products (goods). Enterprises should also be qualified by the recent annual inspection of the market supervision and administration agencies.
3) For the foreign-funded enterprises, they should be qualified by the recent joint annual inspection.
4) Enterprises should have fixed officed space in the regions which has registered in market supervision and administration agencies.
5) Enterprises should establish and implement effective foods quality and safety management regulations
6) Enterprises should cooperate with customs in performing official duties, avoid shrinking the relevant responsibilities.
7) Enterprises should have no violations to laws or regulations.
3. Responsible department
Custom in the place of registration
4. Filing documents
1) Application form of filing (see attachment).
2) Information of institutional setup, departmental responsibilities and occupational responsibilities of agencies which take charge of food safety management.
3) Varieties of food products which intend to sell in the market, and locations of storage.
4) Relevant statement of importing, manufacturing and selling situation of food and cosmetic products of the company in recent two years.
5. Filing procedures
1) Login to “Registration Systems of Imported Food and Cosmetic Importers and Exporters”(the system has been integrated with “Internet+ Custom” platform) and submit the relevant information.
2) Enterprises should submit the prepared documents to local custom offices. Enterprises should be responsible to the integrity, effectiveness and accuracy to the submitted documents, and ensure that relevant personnel on the filled information can be contacted while meeting the case of emergency.